|
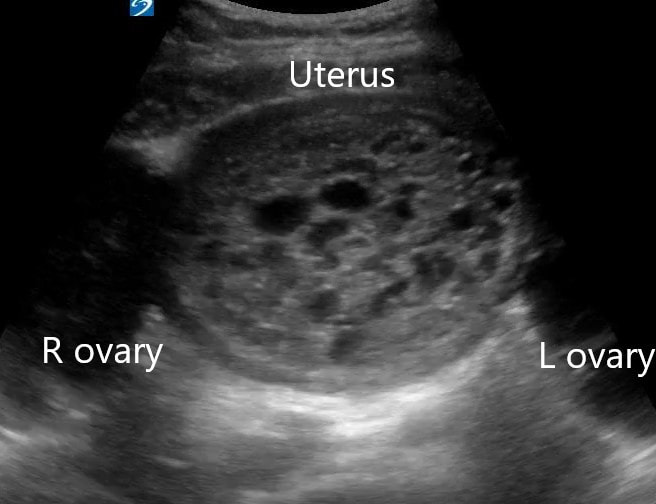
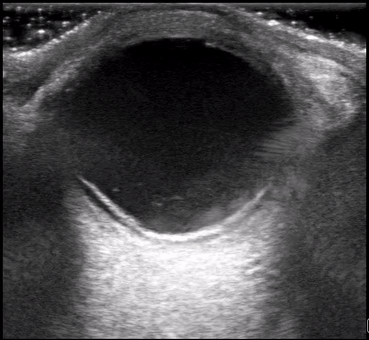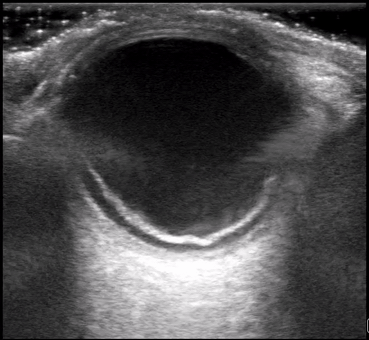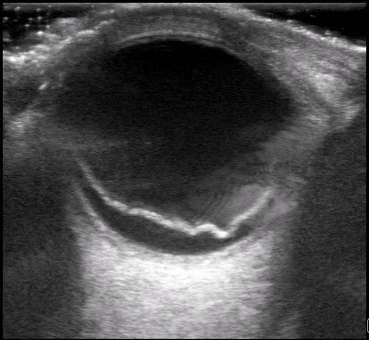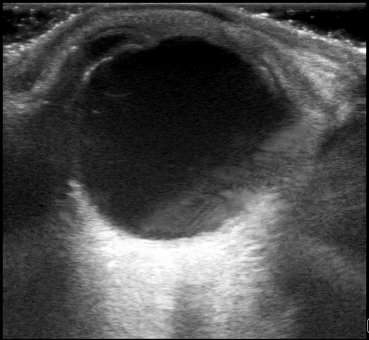
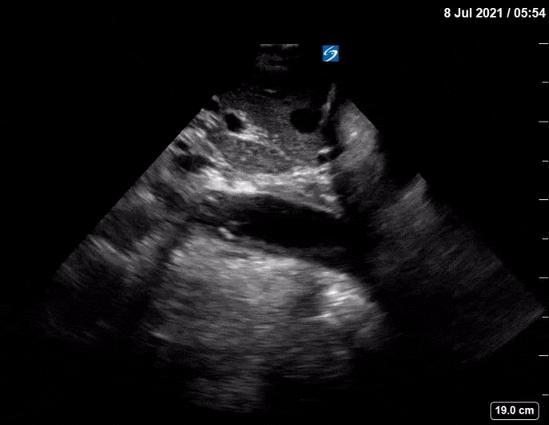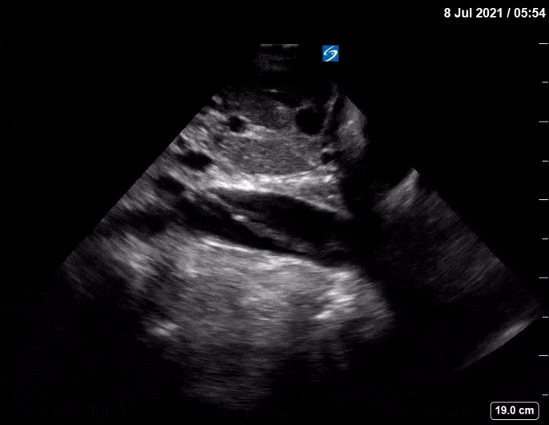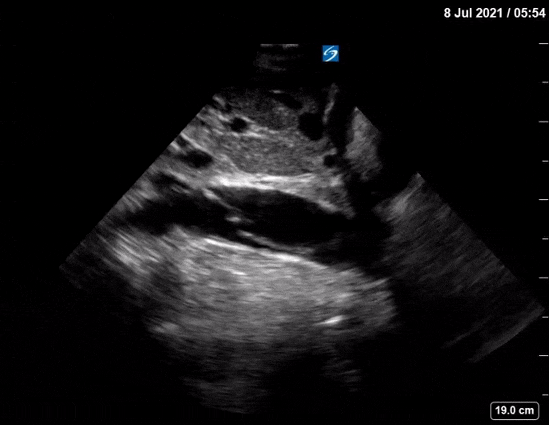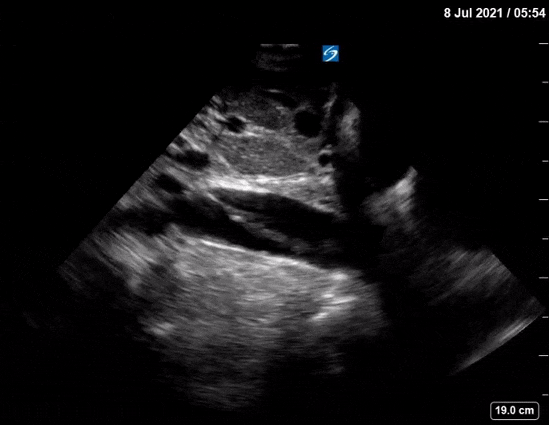
Dr. Winkels diagnosed a molar pregnancy on ultrasound. Note the 'cluster of grapes' or 'snowstorm appearance' within the uterus that is typically described. Another finding in these images are the bilateral enlarged, cystic ovaries. This is referred to as Hyperreactio Luteinalis, and is associated with significantly elevated bHCG (this patient >770, 000) and is seen in approximately 25% of patients with molar pregnancies.
0 Comments
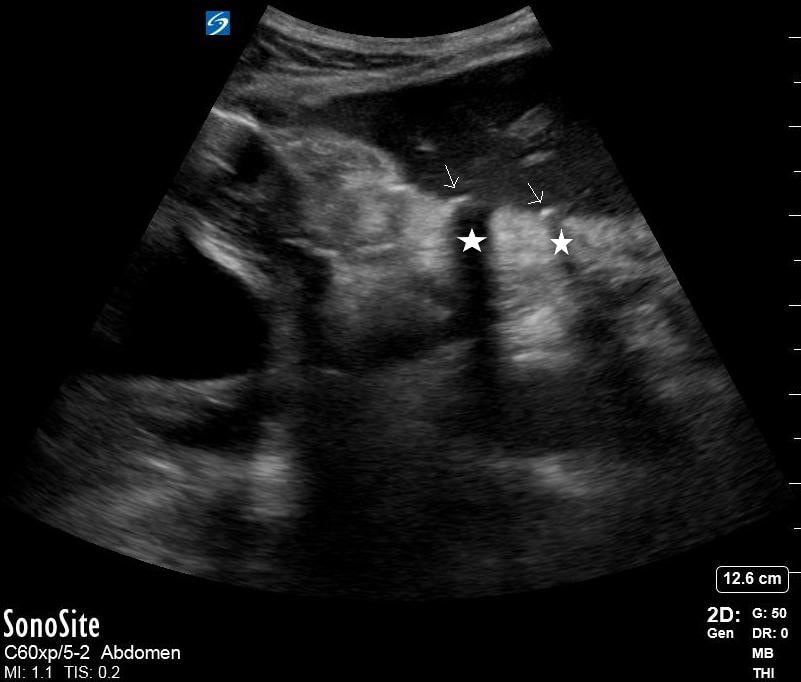
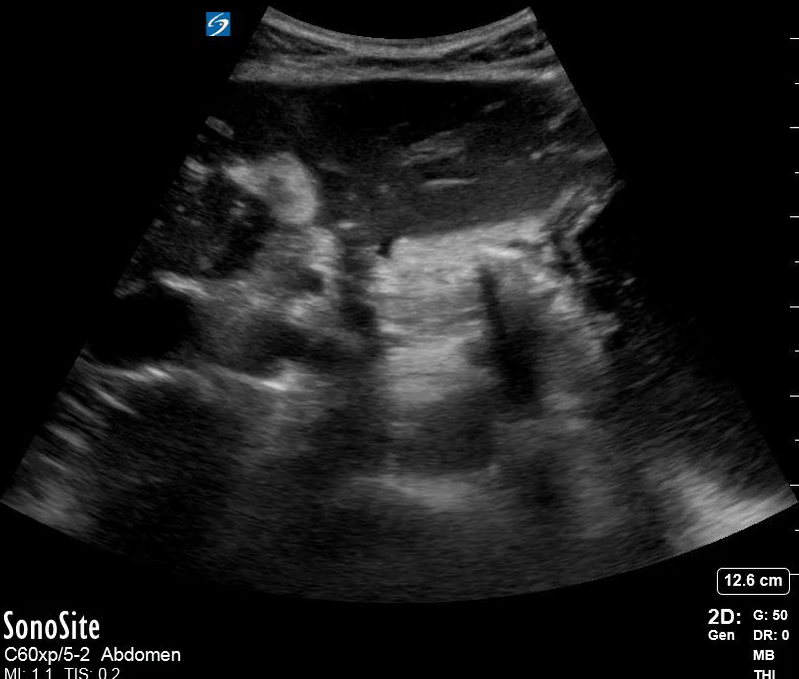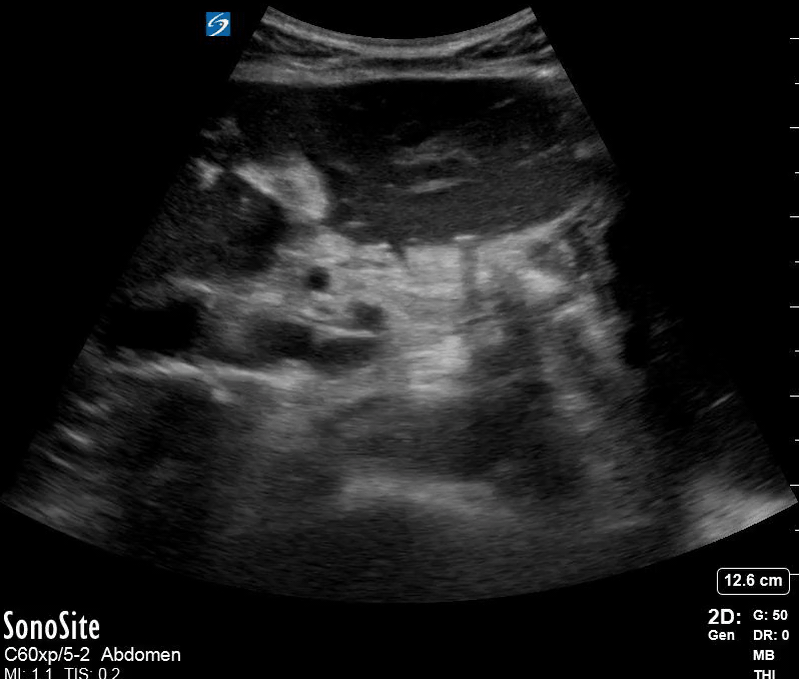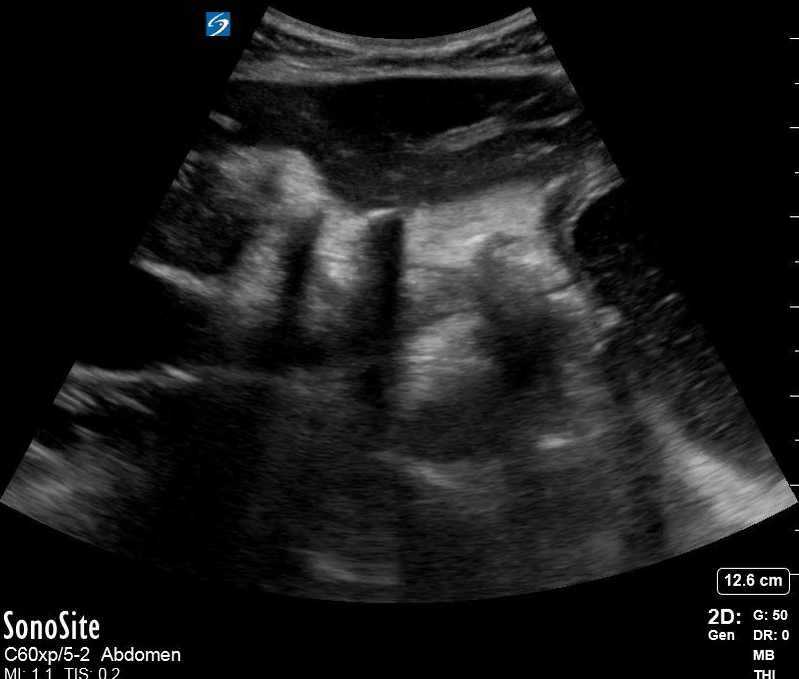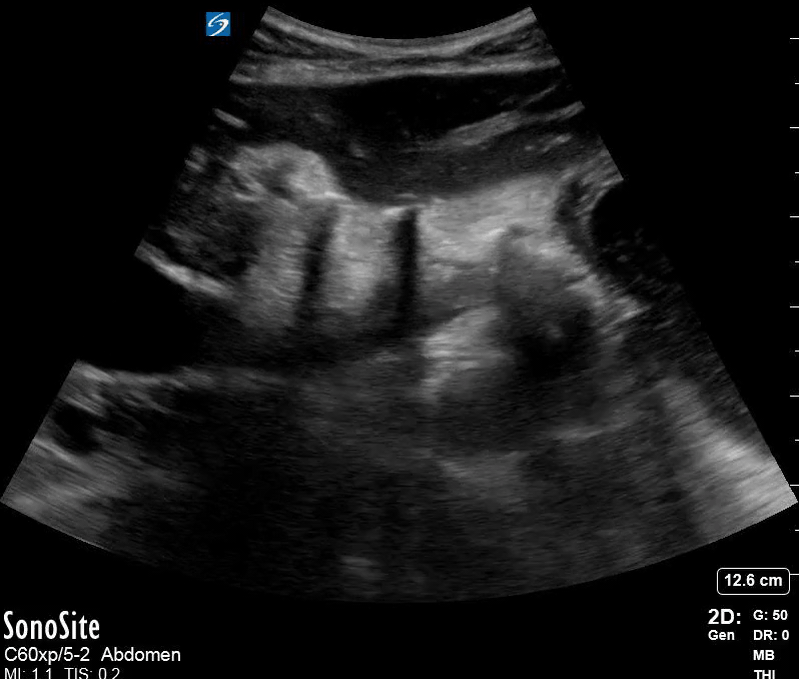
Dr. Lamb picked up on free air from a perforated gastric ulcer in a patient who presented with severe abdominal pain. Note the thin hyperechoic stripes (arrow) with posterior acoustic shadowing (star). In the clip there is also a small amount of free fluid adjacent to the free air.
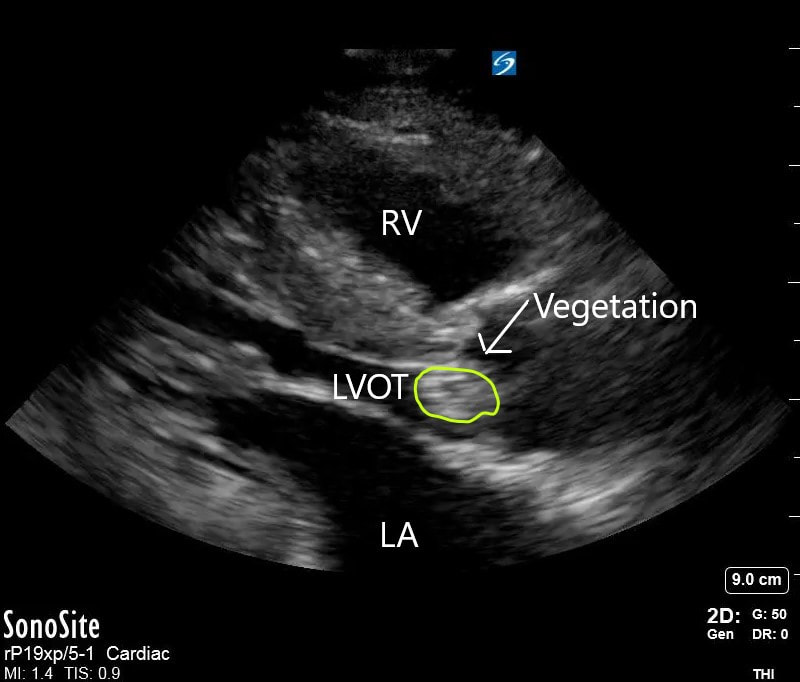
Dr. Lew diagnosed Aortic Valve endocarditis on a patient who presented with vague symptoms and generalized weakness. Her POCUS diagnosis resulted in a complete change in patient management and appropriate therapy for the endocarditis. On the Ultrasound images, note the vegetation attached to the AV leaflets protruding into the LVOT. Here we are again! Drum roll for August’s Ultrasound of the Monthaward, which goes to Dr. Ryan Rees! Please see the fellows for your prize. This patient was a 62 y/o male who presented with new flashers and floaters of the right eye. Visual acuity in the affected eye was 20/70. Dr. Rees astutely grabbed the ultrasound machine, selected the linear probe, adjusted the depth perfectly, and then cranked up the gain appropriately to get a good view into an otherwise deeply anechoic vitreous space. Clip 1 reveals a mobile hyperechoic tissue band that appears to be anchored laterally and floats above the back of the eye. Extraocular movements cause the band to “wiggle” after the eye has finished moving – a phenomenon called “after movement”. Choroidal detachment will look similar but will have very limited after movement. You’ll notice also in Clip 1 that the optic nerve is mostly not visible. The main differential diagnosis for clip 1 is retinal detachment vs. vitreous detachment (which can appear almost identical). The key differentiating factor is that thevitreous detachments do not necessarily anchor to the optic nerve and will exhibit fairly dramatic after movement for that reason (image below for reference). Clip 2 shows the retina peeling of just lateral to the optic nerve, thereby illustrating one ofthe key anchor points that help make the final diagnosis of retinal detachment. The other anchor “point” for the retina is the ora serrata (anterior margin of the retina that interfaces with the ciliary bodies).
Keep the interesting (and high quality) scans coming! Your Ultrasound Fellows, Ian and Alek Welcome to another academic year!
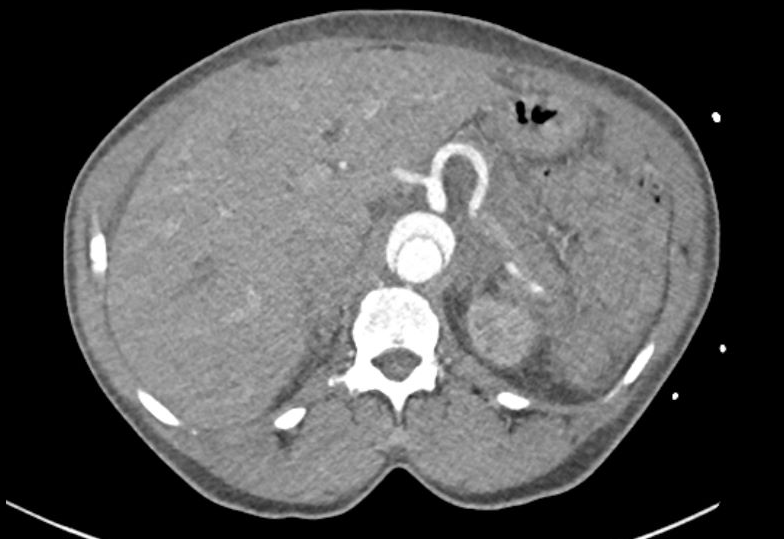
As your new US fellows, along with the Ultrasound Division, we would like to continue the Dr. Greenstein tradition of Ultrasound of the month. Thanks to everyone for the great ultrasound images this past month - it was truly a challenge to pick the single best image of the month. The Ultrasound of the month winner for July is Dr. Kaitlin Parks. Please see the fellows for your prize! Dr. Parks had a great pick up of a type B aortic dissection on a patient with ESRD, HTN and a known pericardial effusion who presented with chest pain after a missed dialysis session. Dr. Parks very appropriately performed a cardiac ultrasound. After imaging the IVC, she noted an irregularity in the descending aorta, consistent with a dissection flap, prompting her to order a dissection protocol CT. The CT confirmed the presence of a type B dissection originating at the distal margin of the left subclavian artery, terminating just superior to the origin of the SMA. The patient was admitted to the ICU and was medically managed. The ultrasound clip below clearly demonstrates a mobile dissection flap within the lumen of the aorta. The image appears to show the dissection flap terminating at the origin of the SMA (as read on the CT scan). CT scan image showing the type B dissection - note that the celiac trunk is supplied by the false lumen! Keep up the great work! Your Ultrasound Fellows, Ian and Alek CONGRATULATIONS to Dr. Jeffrey Ruggeri for March Ultrasound of the Month and CONGRATULATIONS to Dr. Arthur Forbriger for April Ultrasound of the Month!!! Dr. Ruggeri found a large pericardial effusion in a patient with shortness of breath and was able to diagnose them with sonographic tamponade. He wisely used M-mode in parasternal long to assess for early diastolic RV collapse. The patient was admitted to the CCU and had a pericardiocentesis the following day. Strong work! Dr. Forbriger was able to diagnose an incarcerated incisional hernia in a patient with abdominal pain and history of previous incisional hernia. Check out the attached clips! I personally have never seen ultrasound used for this clinical question but clearly it works! Notice the back and forth peristalsis of bowel contents which indicates obstruction. Dr. Forbriger was even able to capture images of the resolved hernia after manual reduction. Very cool! Your awards are in your mailboxes. Keep up the good work and keep the scans coming! |
AuthorWash U EM Ultrasound Faculty and Fellows Archives
November 2021
Categories |
 RSS Feed
RSS Feed